|
Bacterial Susceptibility to Antimicrobial Agents
(F 1 - F 5)
|
|
F
1
SURVEY
ON PENICILLIN SUSCEPTIBILITY OF STREPTOCOCCUS PNEUMONIAE
ISOLATED FROM HUNGARIAN CHILDREN AT A PAEDIATRIC HOSPITAL
IN BUDAPEST
D.
Boriszova, I. Budai, C.L. Marodi, F. Rozgonyi
Pal Heim Children’s Hospital, Hospital of the Ministry
of Home Affairs, Budapest, Semmelweis University, Institute
of Medical Microbiology, Budapest, Hungary
Objective:
To present the penicillin susceptibility of 784 S. pneumoniae
isolates from different samples in Hungarian children during
the period between April 1, 2000 and August 1, 2002.
Methods
and materials: Samples were obtained from children aged
between 1 month and 14 years as follows: blood culture 12;
pleural fluid 5; CSF 4, tympanocentesis and sinus aspirates
48; conjunctiva 8; nasal and throat swabs 707. Susceptibility
tests were performed with 1 mcg oxacillin (Oxoid) screening
disc, and penicillin E-test (AB Biodisk, Sweden).
Results:
The oxacillin disc screening identified 244 (31%) oxacillin
resistant (diameter of the zone of inhibition < 20 mm)
isolates. With penicillin E-test, results differed significantly;
only 8 isolates (1%) appeared to be penicillin resistant
(MIC 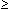 2 mg/L); and 194 isolates (25%) were intermediate resistant
(MIC 0.12-1 mg/L).
2 mg/L); and 194 isolates (25%) were intermediate resistant
(MIC 0.12-1 mg/L).
Conclusion:
Applying the up to date method (E-test) and NCCLS 2000 guidelines,
penicillin resistance of S. pneumoniae isolates appeared
to be much lower than it was predicted by the 1 mcg oxacillin
disc. Consequently, the value of 1 mcg oxacillin disc screening
is questionable.
|
|
|
F
2
STREPTOCOCCUS
PNEUMONIAE AND HAEMOPHILUS INFLUENZAE RESISTANCES
IN SANTIAGO DE COMPOSTELA (SPAIN)
E.
Leon Muiños, P. Ordoñez Barrosa, E. Prieto
Rodriguez, F. Pardo
Perez del Molino Bernal, M.L. Servicio de Microbiologia,
Hospital Clinico
Universitario Santiago de Compostela, Spain
It has
been studied antibiotics resistances for S. pneumoniae
and H. influenzae isolated from 1 January to 30 June
2002; most of them associated with respiratory disease,
in the area of Santiago de Compostela (300.000 hab.), Spain.
Sensitivity patterns obtained from 96 clinical strains of
S. pneumoniae were as follow: penicilin 64.8%; amoxicillin-clavulanate
90.4%; cefotaxime 89.2%; clarithromycin 67.3%; azithromycin
64.6%; telithromycin 100%; ciprofloxacin 64.1 %; ofloxacin
85.3% and levofloxacin 92.8%. Percentages of isolates susceptible
to the antibiotics tested of 89 strains of H. influenzae
were: amoxicillin-clavulanate 100%; cefuroxime 92.68%; cefotaxime
100%; clarithromycin 69.8 %; azithromycin 97.64%; telithromycin
100%; ciprofloxacin 100%; levofloxacin 100%; cotrimoxazole
74.69% and also 19.3% producing B-lactamases. A multiple-antibiotic
resistance pattern was shown by 81.8% of all penicillin
high and intermediate-resistance S. pneumoniae. We
found 64% multiple-resistances for H. influenzae
in those which had betalactamases. Obtained results strongly
suggest the necessity for permanent microbiological monitoring
of development of the resistance to these organisms, due
to frequent use of empirical therapy, almost when new antibiotics
are introduced.
|
|
|
F
3
STUDY
OF PENICILLIN RESISTANT PNEUMOCOCCI ISOLATED IN ROMANIA
BETWEEN 2001-2002
M.
Pana, M. Ghita, I. Rebedea, S. Iacob, O. Dorobat, V. Ungureanu,
R. Papagheorghe, N. Popescu, M. Andrei, S. Botea, G. Bancescu,
I. Nistor
Cantacuzino Institute, Matei Bals Institute, Victor Babes
Hospital, Coltea Hospital,
Emergency Hospital, Carol Davila University, Grigore Alexandrescu
Hospital, Romania
Objective:
To study the antibiotic resistance in pneumococci isolated
last years in Romania.
Methods:
517 strains of Streptococcus pneumoniae coming from
sputum or tracheal aspirate (N=263), blood (N=152), CSF
(N=79) and others (N=23): ear and eye fluid, sinus, were
collected between 2001-2002 at the National Reference Center
for Streptococcus. The isolates were tested for susceptibility
(MICs) to the following antibiotics: penicillin (Pc), erythromycin
(Em), tetracycline (Te), chloramphenicol (Cm), cephalothin
(Kf), cefuroxim (Cxm), cefotaxim (Ctx), amoxicillin (Amx),
trimethoprim/sulfamethoxazole (Sxt), ofloxacin (Ofx), vancomycin
(Va) by standard dilution MIC testing.
Results:
Breakpoints were used as proposed by NCCLS 2002. During
the study period penicillin-resistant strains (55%) of S.
pneumoniae were noted as follows: 65% (MIC50=0.25 mg/l,
MIC90=2 mg/l) in sputum and tracheal aspirate, 38.1% (MIC50=0.12
mg/l, MIC90=1 mg/l) in blood, 10% (MIC50=0.06 mg/l, MIC90=2
mg/l) in CSF and 72.4% in others (MIC50=1 mg/l, MIC90=4
mg/l). The penicillin-resistant strains (coming from sputum
and tracheal aspirate) showed susceptibility to 3 antibiotics:
Amx (100%), Ofx (98.3%), Ctx (86.5%) and resistance to the
following antibiotics: Sxt (74%), Em (45%), Kf (43%), Cxm
(28.7%), Cm (21%). No resistant strain to vancomycin was
found.
Conclusion:
The most efficient drugs against penicillin-resistant pneumococci
were Amx, Ofx and Ctx. In Romania there is an urgent need
for a stronger partnership between clinical medicine and
public health for the surveillance of the antimicrobial
usage.
|
|
|
F
4
CLINIC
AND BACTERIOLOGIC EVALUATION OF COMMUNITY-ACQUIRED PNEUMONIA
(CAP) DUE TO STREPTOCOCCUS PNEUMONIAE
S.
Iacob, I. Rebedea, M. Pana, A. Cristescu, D. Cocioaba, L.
Benea,
C. Marin, M. Ioan
Matei Bals Inst. of Infectious Diseases, Cantacuzino Institute,
Bucharest, Romania
Objective:
To assess the clinical and bacteriologic characteristics
of patients hospitalized with pneumococcal pneumonia in
Infectious Diseases Matei Bals Institute (Romania).
Methods:
Hospital based retrospective study over a 1 year period
(2001-2002).
The patients were diagnosed with CAP according to classical
clinical and radiologic criteria. S. pneumoniae was
identified at hospitalisation, before antibiotic administration,
in sputum samples or hemoculture, from all the patients.
Identification and susceptibility testing of S. pneumoniae
from clinical samples was performed with API bioMérieux
system. The tested antibiotics analyzed were: penicillin,
ceftriaxone, levofloxacin, azitromycin, erytromycin, clindamycin,
trimethoprim/sulfamethoxazole.
Results:
The study included 58 patients between 18-80 years; 60%
of the patients were aged 60 or more. Average length of
stay was 7 days. Risk factors were associated in 50% of
cases (predominently cardiovascular diseases 33% and chronic
obstructive pulmonary diseases 17%). Acute onset with fever,
chills, and pleuritic pain was observed only in 66% of cases.
The pneumonia was complicated in 48% of cases (34% with
pleural effusion and 14% with). 24% of patients showed also
Ig M seroconversion for Mycoplasma pneumoniae (8
patients 14%), Chlamydia pneumoniae (4 patients 7%),
Legionella pneumophila (2 patients 3,5%). The percentages
of S. pneumoniae resistance to the tested antibiotics
were: penicillin 20%, ceftriaxone 9%, azitromycin and
erytromycin 75%, clindamycin 37%, trimethoprim/sulfamethoxazole
100%, levofloxacin 0%.
Conclusion:
Aged patients with cardiovascular diseases and chronic obstructive
pulmonary diseases are at high risk for pneumococcal pneumonia.
In this group of patients hospitalisation is important to
establish the associated pathogens, the antibiotic susceptibility
of S. pneumoniae, and also to follow the clinical
outcome. In our country levofloxacin seems to be the most
appropriate antibiotic in pneumococcal CAP, due to large
spectrum and excellent in vitro activity.
|
|
|
F
5
PHARMACODYNAMIC
OF NEW SLOWLY-RELEASED PHARMACEUTICAL FORM OF CLARITHROMYCIN
FOR PNEUMOCOCCAL PNEUMONIA
N.S.
Yutanova, S.V. Sidorenko, S.V. Yakovlev, S.A. Grudinina
State Scientific Center on Antibiotics; Moscow Medical Academy;
Moscow, Russia
Study
objective: To study pneumococcal susceptibility to Clarithromycin
and plasma concentration of the drug after oral administration
of new slowly-released pharmaceutical form.
Patients
and methods: Clarithromycin blood concentrations after
oral administration of slowly-released pharmaceutical form
at the dose of 500 mg were studied in 12 patients with community-acquired
pneumonia. Susceptibility of freshly isolated S. pneumoniae
strains to Clarithromycin was studied using serial dilution
method. Strain susceptibility was assessed according to
current NCCLS guidelines.
Results:
A total of 214 S. pneumoniae strains isolated from
patients with community-acquired pneumonia in Moscow in
2002 were tested. 85% of the strains were inhibited by Clarithromycin
in concentration of 0.25 mcg/ml or lower. 14% of S. pneumoniae
strains were resistant to Clarithromycin. Half (7%) of resistant
S. pneumoniae strains demonstrated high-level resistance
(MIC 16-32 mcg/ml), the same strains were Clindamycin resistant
(MLSB-phenotype). Another half of
resistant strains showed low level resistance to Clarithromycin
(MIC 1-4 mcg/ml) with retained susceptibility to Clindamycin
(M-phenotype). MIC50 and MIC90
Clarithromycin for S. pneumoniae were 0.032 and 4.0 mcg/ml,
respectively. Clarithromycin average plasma concentrations
in 12 and 24 hours after administration of 500 mg in single
oral dose of slowly-released tablets were 1.71 and 0.95 mcg/ml,
respectively. Clarithromycin blood concentrations in 12
hours after oral administration (50% of dosing interval)
exceeded MIC for 90% of isolated S. pneumoniae strains.
Conclusion:
The results of pharmacodynamic studies of Clarithromycin
slowly-released pharmaceutical form allow predicting good
efficacy of the drug in patients with community-acquired
pneumococcal pneumonia.
|
|
zurück
|